Graduate School of Science and Engineering Applied Chemistry
- Course Outline
- Electrochemical Laboratory
- Laboratory of Physical Chemistry
- Polymer Chemistry Laboratory
- Laboratory of Inorganic Synthetic Chemistry
- Functional Organic Chemistry Laboratory
- Laboratory of Catalysis and Material Design
- Laboratory of Biofunctional Chemistry
- Powder Technology Laboratory
- Transport Phenomena Laboratory
- Biochemical Engineering Laboratory
- Separation and Detection Chemistry Laboratory (Analytical Chemistry Laboratory)
- Molecular Chemical Engineering
- Material System Laboratory
- Biosensing Laboratory
Electrochemical Laboratory
Using electrochemistry to develop novel materials and energy conversion systems
Staff

INABA Minoru
[Professor]
| Acceptable course | |
|---|---|
| Master's degree course | ✓ |
| Doctoral degree course | ✓ |
Telephone : +81-774-65-6591
minaba@mail.doshisha.ac.jp
Office : SC-321
Database of Researchers

DOI Takayuki
[Professor]
| Acceptable course | |
|---|---|
| Master's degree course | ✓ |
| Doctoral degree course | ✓ |
Telephone : +81-774-65-6592
tdoi@mail.doshisha.ac.jp
Office : SC-322
Database of Researchers
Research Topics
- Developing Materials for Batteries and Fuel Cells
- Analyzing Reactions Used in Batteries and Fuel Cells
- Developing New Battery Reaction Systems
Research Contents
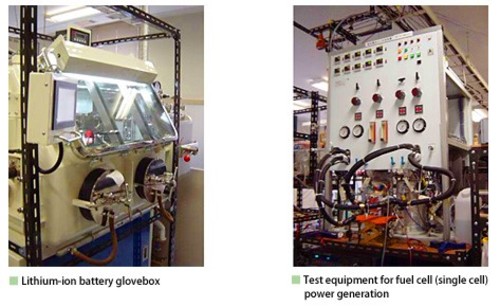
Elucidating the fundamental Mechanism of Electrode Reactions in Lithium Secondary Batteries and Fuel Cells and Developing New Materials
Electrochemical energy conversion systems, such as lithium secondary batteries and fuel cells, are clean, highly efficient systems that can serve as a key solution to effective use of fossil fuels and environmental problems facing our society in the 21st century. Such energy conversion systems can be used as power supplies for hybrid electric vehicles, distributed energy storage systems for electric-load leveling, or cogeneration (or combined heat and power) units for home use. With an aim of achieving higher performance and enabling earlier commercialization of such energy conversion systems, our research focuses on elucidating the fundamental mechanism of electrode reactions used in such systems and developing new materials.
1) Lithium-Ion Batteries:
Large lithium-ion batteries for electric vehicle power sources and for distributed energy storage will need drastic
improvement in energy density, power density, durability, and safety to allow their practical use. To develop such
high-performance batteries, our group is actively engaged in developing silicon negative-electrodes, 5 V class
positive-electrodes and highly stable electrolyte solutions, as well as analyzing the fundamentals of electrode
reactions with in-situ analysis methods, such as Raman spectroscopy and atomic force microscopy (AFM).
2) Fuel Cells:
To allow widespread use of polymer electrolyte fuel cells (PEFCs), our laboratory, in a collaborative research
project with industry and government, is developing core-shell type Pt catalysts that would reduce the use of
platinum in cathode catalysts. Ammonia is a promising hydrogen carrier in the hydrogen energy society. We are also
engaged in developing ammonia-fueled solid oxide fuel cells (SOFCs) operating at high temperatures lower than 500 °C
for use in electric power plants and fuel cell vehicles.
Keywords
- Electrochemical energy conversion
- Lithium-ion batteries
- Fuel cells
- Electrode