Graduate School of Science and Engineering Applied Chemistry
- Course Outline
- Electrochemical Laboratory
- Laboratory of Physical Chemistry
- Polymer Chemistry Laboratory
- Laboratory of Inorganic Synthetic Chemistry
- Functional Organic Chemistry Laboratory
- Laboratory of Catalysis and Material Design
- Laboratory of Biofunctional Chemistry
- Powder Technology Laboratory
- Transport Phenomena Laboratory
- Biochemical Engineering Laboratory
- Separation and Detection Chemistry Laboratory (Analytical Chemistry Laboratory)
- Molecular Chemical Engineering
- Material System Laboratory
- Biosensing Laboratory
Laboratory of Inorganic Synthetic Chemistry
Website of the Laboratory 【In Japanese】Aiming for new materials, new properties and new synthetic processes
Staff
![Masaki KATO [Professor]](/istc/attach/page/TECHNOLOGY-PAGE-EN-59/152453/resize-file/Masaki_KATO.jpg)
KATO Masaki
[Professor]
| Acceptable course | |
|---|---|
| Master's degree course | ✓ |
| Doctoral degree course | ✓ |
Telephone : +81-774-65-6686
makato@mail.doshisha.ac.jp
Office : SC-207
Database of
Researchers

OTA Hiroto
[Associate Professor ]
| Acceptable course | |
|---|---|
| Master's degree course | |
| Doctoral degree course | |
Telephone : +81-774-65-6690
hirota@mail.doshisha.ac.jp
Office : SC-208
Database of Researchers
Research Topics
- Fabrication of magnetic metal/ceramic composites used for electric applications
- Development of oxide thermoelectric materials
- Fabrication of carbon nano fiber (CNF) or carbon nano tube (CNT) dispersed engineering ceramics
- Fabrication of high-tempertaure engineering ceramics usable around 1,500~1,600°C
- Fabrication of dense bio-ceramics using Fused Deposition Modeling (FDM) type 3D printer
- Fabrication of dense ZrO2-Al2O3 based ceramics with high mechanical properties by pressure-less sintering
- Preparation of antibacterial ceramic powders, such as ZnO and TiO2
- Effect of elemental substitution on magnetic interactions in layered copper oxide
- Effect of elemental substitution on magnetism in indium (In), copper (Cu) oxide with its particularly low-dimensional structure
- Elemental substitution effect and microscopic electronic properties of pyrochlore oxide
- Synthesis and physical properties of iron-based superconductive compounds and properties
- Synthesis and physical properties of titanium oxide as a new transparent electrode
Research Contents (Prof. Ken HIROTA)
<1> Production and characterization of fine particle (nanometer-sized) powders.
We use new methods of oxide powder production of spinel compounds such as (Mn-Ni)Fe2O4 and (Mn-Ni-Zn)Fe2O4, and semi-conducting ZnO and TiO2, and its related compounds, and examine the powder properties (particle size, crystalline phase, phase transition, specific surface area, and antibacterial activity under dark conditions etc). High density ceramics are also produced using various sintering methods, and then their microstructure and electric and magnetic properties are evaluated.
<2> Production and evaluation of high-density ceramics-based monolythic and composite materials using high pressure compacting, high-temperature/high-pressure and microwave-sintering processes.
- Cold isostatic pressing (CIP: max 392 MPa (4,000 kg/cm2))
- Hot isostatic pressing (HIP: 2,000°C, 196 MPa (2,000 kg/cm2))
- Pulsed Electric-Current Pressure Sintering (PECPS, or Spark Plasma Sintering: SPS: 1,900°C, 30 - 50 MPa (300 - 500 kg/cm2))
- Microwave-sintering (increasing temperature rate of 30~50°C/min, 1,300°C, in N2)
If inorganic materials show poor sinterability and it is difficult to evaluate their properties or put it to practical use, the materials are densified by the above-mentioned process, and then the properties of the dense sintered monolythic and composite material are evaluated.
<3>
Production of inorganic compound powders with high
melting points such as nitrides, borides, and carbides introducing self-propagating high-temperature synthesis
(SHS) and their powder characterization, and production and evaluation of high-density ceramics obtained using
process 2 above.
We synthesize the inorganic compound powders with high melting points using SHS, and aim to produce the dense
bulk materials with the same composition during the SHS process. Then, we characterize these powders and
evaluate the mechanical, electrical, and magnetic properties of the bulk materials in relation to their
microstructures.
<4> Nano composites
- Fabrication and evaluation of nano-composites, in which carbon nano tube (CNT; one of the novel carbon allotropes that leads to nano technology) or the similar carbon nano fibers (CNF) are dispersed homogeneously into the ceramic matrix.
- Production and properties-evaluation of magnetic nano-composites consisting of magnetic metals particles and magnetic ferrite materials, that reveal superior electrical and magnetic properties at high frequencies.
<5> Production of new functional materials
- Production of zinc oxide ZnO powder using hydrothermal treatment and anatase-type titanium oxide α-TiO2 using solid state reaction. These powdes show sustainable antibacterial properties under dark conditions.
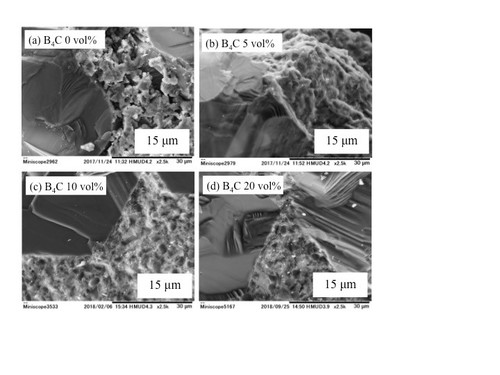
Microstructural images (SEM) ofdense diamond/SiC/B4C composites.
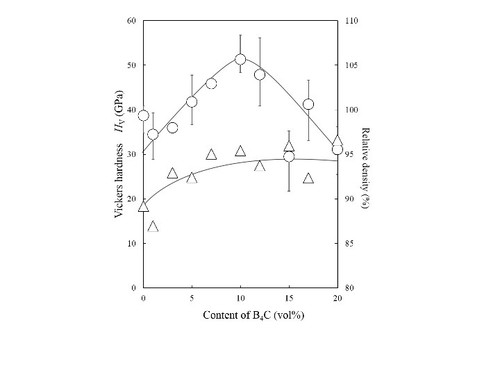
Vickers hardness Hv of diamond/SiC/B4C composites: very high Hv =50 GPa is achieved.
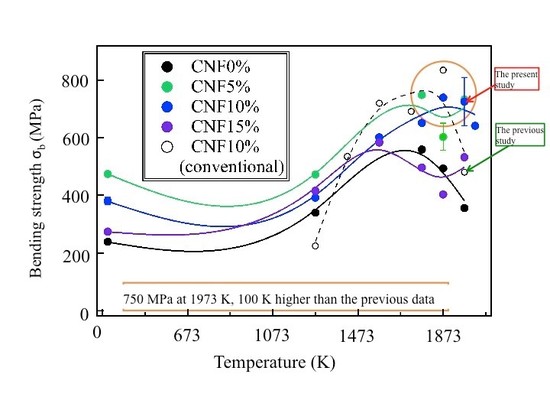
High-temperature bending strength σb of (B4C/CNF) composites:
high σb is attained at1,400~1,700°C (1,673~1,973 K).
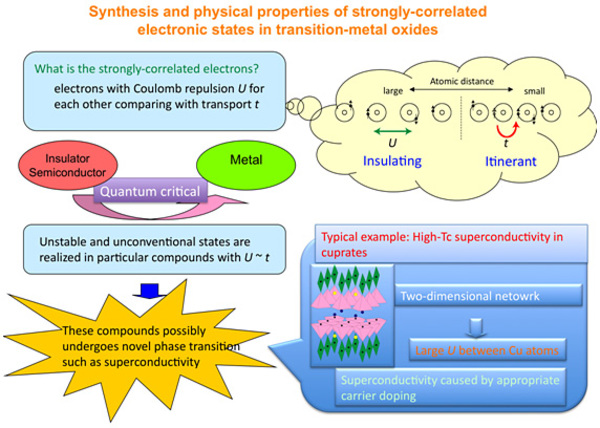
Research Contents (Associate Prof. Masaki KATO)
Superconductivity is a phenomenon whereby perfect diamagnetism (property which internally
cancel the external magnetic field) called Meissner effect occurs with zero electric resistivity. The applications
are too numerous to mention (e.g. development of ultra-strong magnetic fields, lossless power transmission,
linear-motor trains and other modes of transport that use magnetic levitation, power storage, nuclear fusion), but
the development of materials that become superconductive at high temperatures remains a major issue. To this end,
a fundamental elucidation of the mechanisms regarding emergence of superconductivity is necessary.
Notably, it is becoming clear that the dimensionality of the crystalline structure are closely linked to
superconductivity and magnetism since the recent discovery of high temperature oxide superconductors and their
related compounds. This strong association between structure and electron properties (conductivity and magnetism)
is particularly noteworthy in inorganic compounds including transition-metals, and this stems from a much stronger
correlation (strong electron correlation) in solids than in ordinary materials. This is a major topic in
properties research of interest from both an experimental and theoretical viewpoint. For example, it can be said
that all unique electric and magnetic phenomena in transition-metal compounds (such as materials that transition
from metal to insulator at a certain temperature, itinerant electron magnets in which the electrons responsible
for electrical conductivity also display magnetism, and heavy electron systems of compounds including rare metals
in which the effective mass of electrons increases up to 100-1,000 times than normal) are based on strong electron
correlation.
However, research on strong electron correlation in solids is still in the early stages, and theoretical
discussion is extremely difficult; thus a fundamental understanding will require consolidation of more
experimental knowledge.
Thus, this laboratory synthesizes such electrically and magnetically unique materials. Specifically, we produce
layered inorganic ceramic compounds controlled materially and structurally on a nanoscale by introducing various
atoms and molecules between layers in layered transition-metal compounds. We then analyze their structure using
X-ray diffraction, electron microscope observation, and the like, and evaluate their physical properties using
such measurements as magnetic susceptibility/electrical resistivity measurement, nuclear magnetic resonance (NMR)
measurement, and neutron diffraction, in order to come to an understanding on a nanoscale of the various phenomena
(or quantum criticality) based on superconductivity or electron correlation or the like. The knowledge gleaned is
fed back into the synthesis, with the ultimate aim of producing new functional inorganic compounds.
<1> Metal-insulator crossover in transition-metal oxides with pyrochlore-type structure
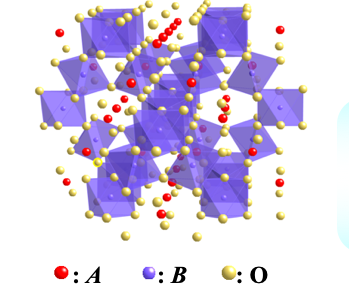
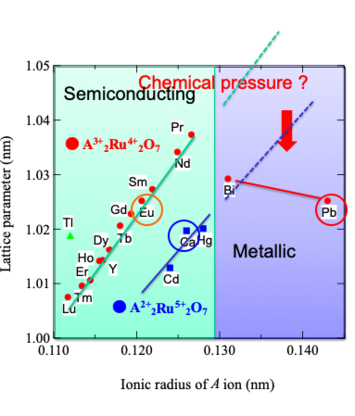
Pyrochlore oxides constitute a large family of transition metal oxides They have a general formula of A2B2O7, where A is usually a larger cation such as lanthanoid series, and B is a
smaller transition metal. Both A and B ions individually form a three dimensional sub-lattice consisting of
corner-shared tetrahedra. When the exchange interaction in the sub-lattice is antiferromagnetic, the magnetic
moments are expected to be geometrically frustrated. As a result, the system shows a spin-glass behavior which
has been found in several pyrochlores or a disordered ground state instead of a magnetically ordered state.
Electric and magnetic properties of pyrochlores with B sites of 4d elements are especially attractive, since the
4d electrons generally indicate the variety of localized and itinerant behaviors10-19). For example, the bismuth ruthenate Bi2Ru2O7 is metallic and Pauli paramagnetic with a nearly temperature-independent
resistivity9), while the rare earth ruthenates Ln2Ru2O7 (Ln represents lanthanoids from Pr to Lu) are all semiconductors with
localized magnetic moment on the ruthenium atoms. In order to clarify the transport behaviors of pyrochlore
ruthenates near the boundary of the metal-insulator crossover, detailed investigations of the physical
properties have been performed for the Ln-substituted system Bi2-xLnxRu2O715). Although Pb2Ru2O7-δ is known to show a Pauli
paramagnetism with a metallic behavior similar to Bi2Ru2O7, there have been a few reports on
Ln-substituted system Pb2-xLnxRu2O7-δ. In this article, our studies for
the synthesis and physical properties of Pb2-xEuxRu2O7-δ solid solution are reviewed21),22).
Especially, we report Pb-Eu system from viewpoints of (1) magnetic and electric properties, (2) microscopic
structural parameters and (3) strongly-correlated electron system behaviors. In addition, our new results of
synthesis under high-pressure oxygen for Pb2-xCaxRu2O7-δ system are also reported.
Schematic view of pylochlore-type structure with chemical formula of A2B2O7
Relationship between lattice parameter and ionic
radius of A-site in pyrochlore-type ruthenates
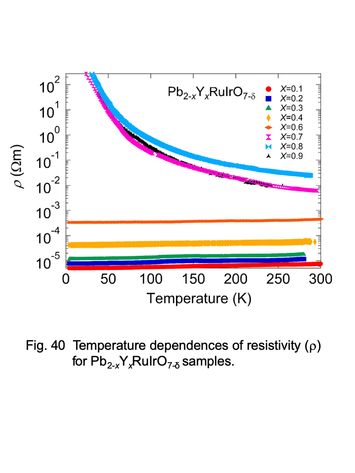
Temperature dependences of electric resistivity for
pyrochlore-type ruthenates, Pb2-xYxRuIrO7
<2> Elemental substitution effects on physical properties of Cu-based oxides with low-dimensional structure
Low-dimensional magnetic compounds, in which magnetic atoms form the one- or two-dimensional (1-D or 2-D)
structure, usually show a variety of phenomena such as quantum spin effect, spin frustration and
superconductivity. These phenomena are caused by the enhancement of quantum fluctuation in low-dimensional
structure. Thus, it is a key role to elucidate the physical properties of low-dimensional compounds with the
quantum fluctuation effect.
CuGeO3 consists of 1-D CuO2 chains
formed by the edge-sharing CuO6 octahedra and separated by Ge ions. Because
of this lattice structure, the magnetic properties of CuGeO3 are shown to
be well described by the 1D antiferromagnetic Heisenberg model with S = 1/2 spins. Moreover, this material
undergoes Spin-Peierls transition at a temperature TSP ≈ 15 K. Spin-Peierls
transition is the magnetic transition with the lattice distortion, where the period of the lattice has just
doubled due to the dimerization of neighboring spin couplings. This spin-lattice coupling is similar to the
Peierls transition, the charge-lattice coupling in 1-D metals.
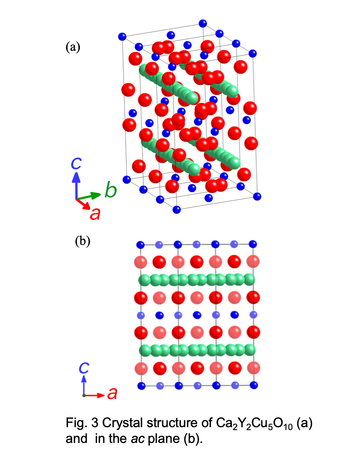
The crystal structure of Ca2+xY2-xCu5O10 consists of 1-D Cu-O chains formed by the edge-sharing CuO4 squares. The end-material Ca2Y2Cu5O10 without holes shows an antiferromagnetic ordering of the Cu2+ moment below 29.5 K with ferromagnetic coupling along the chain. This 1-D
Cu-O chains have two kinds of magnetic interactions, Cu-O-O-Cu superexchange (J1) with almost 180° angle and Cu-O-Cu superexchange (J2) with nearly 90° angle. Since J1 and
J2 have opposite sign, i.e., antiferromagnetic and ferromagnetic, Cu spins
should show the spin-frustration. Because of this feature, it is expected that the magnetic ground state crosses
over from a Néel state to a new type of short-range-ordered state as a function of hole concentration. The
change in magnetic superexchange in this higher hole concentration regime is supposed to be an important part of
the understanding of the CuO2 planes in cuprate.
In this study, the author reports the magnetic properties of CuGe1-xAlxO3 (0.0≦x≦0.4), Cu1-xCoxGeO3 (0.0≦x≦0.12), Ca2Y2-xSrxCu5O10 (0.0≦x≦0.5) and Ca2Y2-xMgxCu5O10 (0.0≦x≦0.4) compounds.
Schematic vies of crystal structure for Ca2Y2Cu5O10 phase with low-dimensional structure.
Powder XRD patterns for Ca2Y2-xMgxCu5O10 solid solutions.
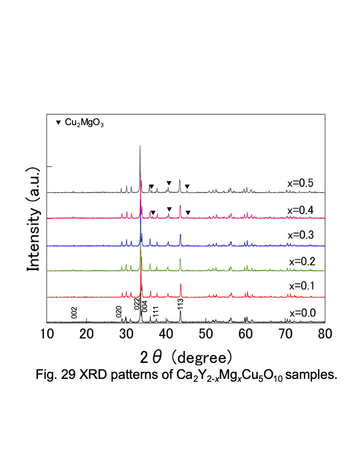
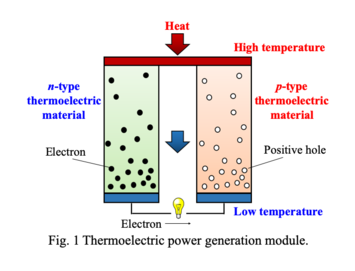
<3> Chemical and thermoelectric properties of transition-metal oxides with such as double-perovskite and low-dimensional structure
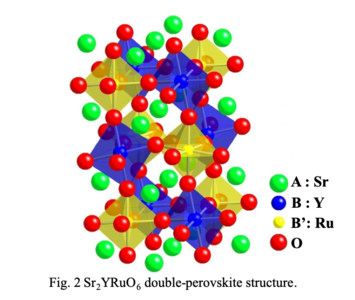
Double-perovskite compounds exhibit various physical properties due to strongly-correlated electron behaviors
such as giant magnetoresistance, negative thermal expansion, multiferroics and superconductivity. Sr2YRuO6 compound with double-perovskite
structure is reported to show high Seebeck coefficient (S = -475 μV/K) and low thermal conductivity (κ = 0.4
W/mK), which are suitable properties for a thermoelectric material. Its electrical conductivity (σ = 0.045 S/cm)
is not enough, however, to increase the dimensionless figure of merit (ZT = S2σT/κ), which is related to the efficiency of a thermoelectric device for
electricity generation. In this paper, the author has studied chemical and thermoelectric properties of Sr2YRuO6 compounds to improve their
electrical conductivity leading to higher value of the thermoelectric figure of merit.
Polycrystalline samples of Sr2-xLaxYRuO6 and S2Y1-xPrxRuO6 were prepared by solid-state
reaction (SSR) at 1523-1673 K. In order to increase relative density of the material, obtained samples were
exposed to pulsed electric-current pressure sintering (PECPS). Samples were evaluated by X-ray diffraction
(XRD), magnetic susceptibility, and thermoelectric property measurements. From XRD measurements, single phases
were obtained by both methods of SSR (0 ≤ x ≤ 0.3) and PECPS (0 ≤ x ≤ 0.2). Magnetic susceptibility measurements
indicated that the valence of Ru decreases with increasing La or Pr content x. Electrical conductivity was found
to be enhanced by the elemental substitution and the densification, leading to maximum value (10.25 S/cm) for
Sr1.7La0.3YRuO6 synthesized by PECPS. The values of Seebeck coefficient for all the sample
were negative and the absolute value |S| was found to be maximum (-473.57 μV/K) for Sr2YRuO6 obtained by SSR. Thermal
conductivity measurements showed 0.41-2.85 W/mK for all samples. The calculated ZT was 1.58×〖10〗^(-2) at most for Sr2Y0.8Pr0.2RuO6 by SSR method.
Conceptual scheme of thermoelectric device.
Crystal structure of double-perovskite compound,
Sr2YRuO6 with n-type thermoelectric
property.
Keywords
- Carbon nano fibers (CNF)
- Nano powder, Nano-composites
- Electronic ceramics
- Engineering ceramics
- Quantum critical phenomena
- Superconductivity
- Low-dimensional magnetism
- Strongly-correlated electron system
- Metal-insulator transition