Graduate School of Science and Engineering Applied Chemistry
- Course Outline
- Electrochemical Laboratory
- Laboratory of Physical Chemistry
- Polymer Chemistry Laboratory
- Laboratory of Inorganic Synthetic Chemistry
- Functional Organic Chemistry Laboratory
- Laboratory of Catalysis and Material Design
- Laboratory of Biofunctional Chemistry
- Powder Technology Laboratory
- Transport Phenomena Laboratory
- Biochemical Engineering Laboratory
- Separation and Detection Chemistry Laboratory (Analytical Chemistry Laboratory)
- Molecular Chemical Engineering
- Material System Laboratory
- Biosensing Laboratory
Functional Organic Chemistry Laboratory
Staff

KITAGISHI Hiroaki
[Professor]
| Acceptable course | |
|---|---|
| Master's degree course | ✓ |
| Doctoral degree course | ✓ |
Telephone : +81-774-65-7442
hkitagis@mail.doshisha.ac.jp
Office : SC-423
Database of Researchers
KODERA Masahito
[Professor]
| Acceptable course | |
|---|---|
| Master's degree course | ✓ |
| Doctoral degree course | ✓ |
Telephone : +81-774-65-6652
mkodera@mail.doshisha.ac.jp
Office : SC-323
Database of Researchers
Research Contents
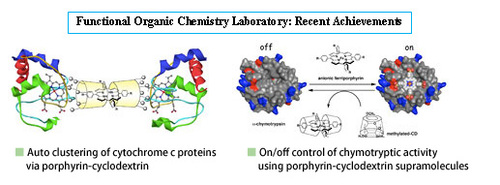
Research on supramolecular chemistry
Supramolecule is the term for a noncovalent molecular assembly of multiple molecules that displays functions different to those of the original comprising molecules. At the Functional Organic Chemistry Laboratory, we conduct research on supramolecular chemistry from various perspectives.
Chemistry of protein supramolecules
Within living organisms, many proteins function to sustain life. Some of these proteins function independently, but
most form complexes (supramolecular complexes) of homologous or heterologous proteins by way of noncovalent binding
interactions and display their vital function only through such binding. In short, weak interactions of noncovalent
binding (as opposed to covalent binding) are used sophisticatedly in the natural world in order to reversibly
control the binding of proteins according to the circumstances.
Since the concept of supramolecular chemistry was propounded by Jean-Marie Lehn (who won the Nobel Prize in
Chemistry 1987) and others, it has been developed using synthetic small molecules such as crown ether and
cyclodextrin. Many researchers are still fascinated with supramolecules, and research is continuing. At the
Functional Organic Chemistry Laboratory, we focus on massive biomolecules such as proteins as one unit of the
supramolecule and our challenge is to control chemically the various molecular aggregation processes that occur
within organisms. Function is controlled in vivo by protein interactions, and if we can replicate this
synthetically, various innovative medical applications can be expected. In addition, we use supramolecular chemistry
in the production of highly biocompatible nano materials using proteins, nucleic acid, cells, and various other
materials.
Our protein research is expanding greatly as part of collaborative research with the Doshisha Women's College of Liberal Arts, Faculty of Pharmaceutical Sciences, and this research theme is expected to be of great interest in the future.
Chemistry of porphyrin-cyclodextrin supramolecules
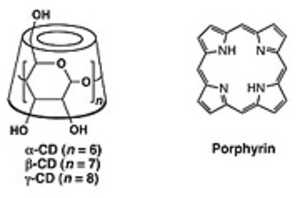
Cyclodextrins (CD) contain six to eight glucopyranose units in a ring, creating a cylinder shape, and are called α-CD, β-CD, and γ-CD respectively. Various molecules can be inserted (included) into the ring cavity by way of noncovalent binding, and thus CDs are renowned for being pioneering within the supramolecular chemistry field. The interior of CD dissolved in water is a highly hydrophobic space, despite being in water, and the inclusion of molecules in water leads to various phenomena that do not occur in uniform aqueous solutions. This CD-regulated environment has brought to light a number of very interesting supramolecules.
Porphyrin is the generic term for large π conjugated compounds that form pigment components that are found
abundantly in nature. It is possible to insert various metal ions in the cavity; and numerous different functions
are expressed depending on the type of metal. Porphyrin is well known as the coenzyme for hemoglobin in human blood.
We have identified various interesting supramolecules, including 1:2 inclusion complexes formed from O-methylated
β-CD (TMe-β-CD) and anionic meso-tetrakis(4-sulfonatophenyl)porphyrin (TPPS). These inclusion complexes are
extremely stable, and so large that the coupling constant was almost impossible to be determined. Through
experiments using our micro-calorimeter, we discovered that major negative enthalpy change is seen when these
inclusion complexes are formed and therefore proved that great stability can be obtained mainly driven by van der
waals interaction.
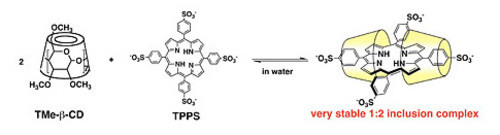
However, not only are these inclusion complexes stable: importantly, they also isolate TPPS completely from water. This "separation of porphyrin from water" is an extremely common phenomenon in the natural world, whereby globin proteins of hemoglobins or myoglobins extract porphyrin-iron complexes (hemes) from the external water phase. Thus, we predicted that TMe-β-CD could be an alternative to the globin proteins. Based on this hypothesis, we started looking at cyclodextrin protein model synthesis bearing in mind the structures of hemoglobin and myoglobin. More specifically, we have designed and synthesized Py3CD molecules by linking two TMe-β-CD units with a pyridine ring, and coordinting nitrogen atoms to the TPPS central metal.
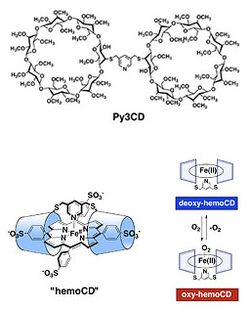
By mixing this Py3CD with Fe (III) TPPS in water, and reducing to Fe (II) using a reducing agent, the oxygen molecules in water bind reversibly to the central Fe (II). This function is very similar to that of hemoglobin or myoglobin. In water, the catalysis of the water normally induces an oxidation reaction of oxygen molecules from Fe (II) to Fe (III) and oxygen binding capacity is lost, but the inclusion of Py3CD markedly interferes with the proximity of water to Fe (II), and the adsorption and desorption of oxygen molecules in water is remarkable. Complexes that can bind oxygen in water like this have never before been discovered. We have called such supramolecular complexes "hemoCD." Capturing oxygen from water is essential in the functioning of synthetic blood. And, at the Functional Organic Chemistry Laboratory, we are researching these hemoCD with a view to implement real-life applications such as synthetic blood and oxygen storage materials.
Keywords
- Supramolecular chemistry
- Proteins
- Porphyrin
- Cyclodextrin
- Enzyme model
- Thermodynamics